Research Questions
-
What are the ubiquitin-mediated signalling events that limit the damage caused by misfolded protein accumulation under acute stress in healthy cells?
-
How does proteostasis network dysregulation contribute to establishment and maintenance of senescent cellular states?
We use a multi-disciplinary experimental approach, with an emphasis on employing the appropriate tool(s) for the job, and developing new tools whenever required. Our current focus includes:
- multi-dimensional proteomics to quantify misfolded protein ubiquitylation and aggregation in diverse biological samples.
- live-cell imaging sensors to measure dynamics of biomolecular condensates with single-molecular resolution.
- 3D in vitro models (e.g., organoids) to model cellular senescence under more physiological settings.
Current Research
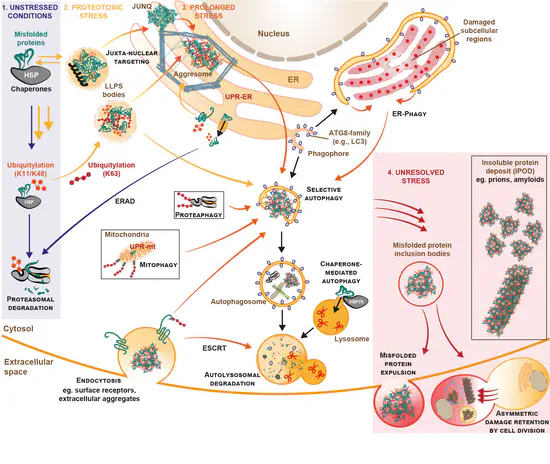
Decoding ubiquitin chain signals in physiologic and stressed states
Although Lys48-linked ubiquitin is the canonical signal for protein clearance by the proteasome, ‘alternative’ ubiquitin signals play critical yet poorly-characterised roles in misfolded protein clearance—especially under acute stress. Using linkage-specific ubiquitin probes and perturbation tools, we are uncovering how these additional ubiquitin signalling events maintain proteome robustness in a range of physiologic and stressed cellular contexts.
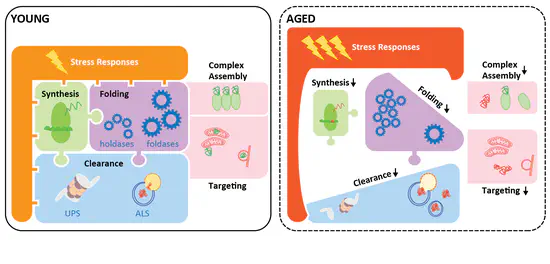
Understanding the interplay between loss of proteostasis and cellular senescence
Loss of proteostasis and cellular senescence are two well-established hallmarks of “normal” ageing, and are linked to almost all major ageing-associated chronic diseases. We are systematically investigating the inter-dependence of these two ageing hallmarks, with the goal of identifying proteostasis network vulnerabilities that could be exploited therapeutically to modulate the senescent cellular state.