Decoding ubiquitin chain signals in physiologic and stressed states
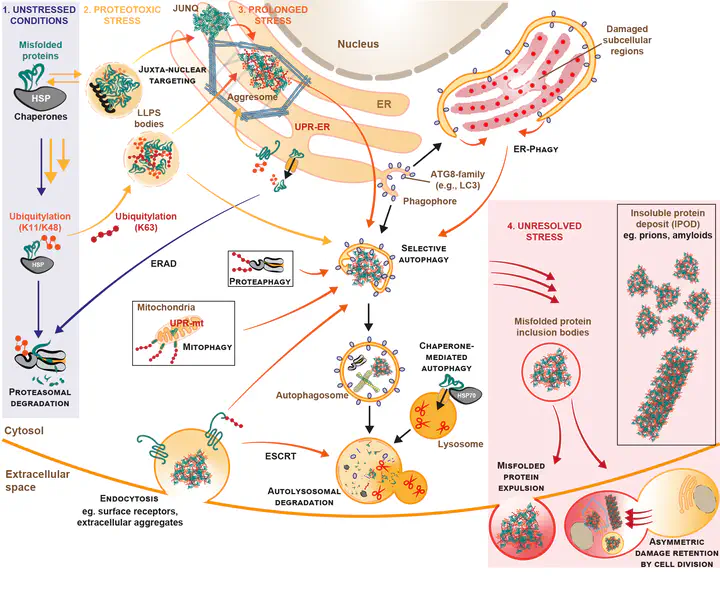 Image credit: Johnston & Samant (2021), FEBS J
Image credit: Johnston & Samant (2021), FEBS J
Although Lys48-linked ubiquitin is the canonical signal for protein clearance by the proteasome, ‘alternative’ ubiquitin signals play critical yet poorly-characterised roles in misfolded protein clearance—especially under acute stress. Using linkage-specific ubiquitin probes and perturbation tools, we are uncovering how these additional ubiquitin signalling events maintain proteome robustness in a range of physiologic and stressed cellular contexts.
Current projects on ubiquitin signalling include:
Impact of specific ubiquitin linkages in misfolded protein clearance
- Project Leads: Harvey Johnston; Richard Odle
- Collaborators: Yogesh Kulathu; Helle Ulrich; Gabriele Kaminski-Schierle; Kirby Swatek
Nascent proteome quality control during dendritic cell activation
- Project Lead: Estelle Wu
- Co-PI: Michelle Linterman
- Collaborators: Ed Roberts